Extraction of iron from its ore is the third and the penultimate process in the Metallurgy. The extraction of metals and its isolation occurs over a few major steps:
- Concentration of Ore
- Extraction of metal from concentrated Ore
- Purification of the metal
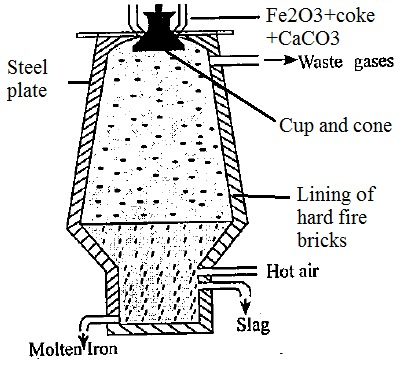
It’s a long process which begins with Concentration along the roasting process known as calcination. Concentration removes the water and other volatile impurities such as sulphur and carbonates. This concentrated ore is mixed with limestone (CaCO3) and Coke and fed into the blast furnace from the top. It is in the blast furnace that extraction of iron occurs. The extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag.
The purpose of a Blast Furnace is to reduce the concentrated ore chemically to its liquid metal state. A blast furnace is a gigantic, steel stack lined with refractory brick where the concentrated iron ore, coke, and limestone are dumped from the top, and a blast of hot air is blown into the bottom. All the three ingredients are crushed into small round pieces and mixed and put on a hopper which controls the input.
