Aluminium is a soft, silvery-white, corrosion-resistant metal. It is the most abundant metal in the earth’s crust as it makes up 8% of the crust and it is the third most abundant element after oxygen and silicon. Bauxite ore (Al2O3.xH2O) is the major source of aluminium till date which is a mixture of hydrated aluminium oxide.
Aluminium can also be recovered from cryolite (Na3AlF6) and alunite. It is also found in gemstones such as garnet, topaz and chrysoberyl. The chemical symbol of this metal is Al. In the boron group with symbol Al, aluminium is a chemical element and is the most commonly used non-ferrous metal.
Aluminium Ore
Ores of Aluminium
Aluminium is a highly reactive metal, belonging to the IIIA group of the periodic table. In nature, aluminium is found in the form of its oxide in its ores. The important ores of aluminium are
- Bauxite – Al2O3.2H2O
- Corundum – Al2O3
- Cryolite – Na3AlF6
Metallurgy of Aluminium
Aluminium is mostly extracted from its bauxite ore. Dressing of ore: The ore is crushed and pulverized.
Concentration of ore
The bauxite ore contains ferric oxide and silica as impurities. It is first concentrated by gravity separation of ferric oxide impurities by the process of magnetic separation. The ore is then concentrated by chemical process.
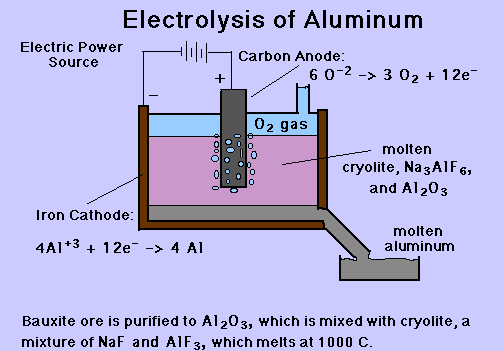
Bauxite is the name given to aluminium ore. To generate the oxide of aluminium from bauxite ore is purified, a white powder form which aluminium can be extracted. Aluminium oxide has a very high melting point of more than 2000 ° C so melting it would be costly. Aluminium oxide in water does not dissolve, but in molten cryolite, it dissolves.
Pure aluminium is a silver-white metal with many desirable features. It’s light, non-toxic, non-magnetic, and non-sparking. It’s a bit ornamental. It’s created, machined, and cast readily. Aluminium in pure state is soft and lacks strength, but it has very helpful characteristics for alloys with tiny quantities of copper, magnesium, silicon, manganese and other components.
